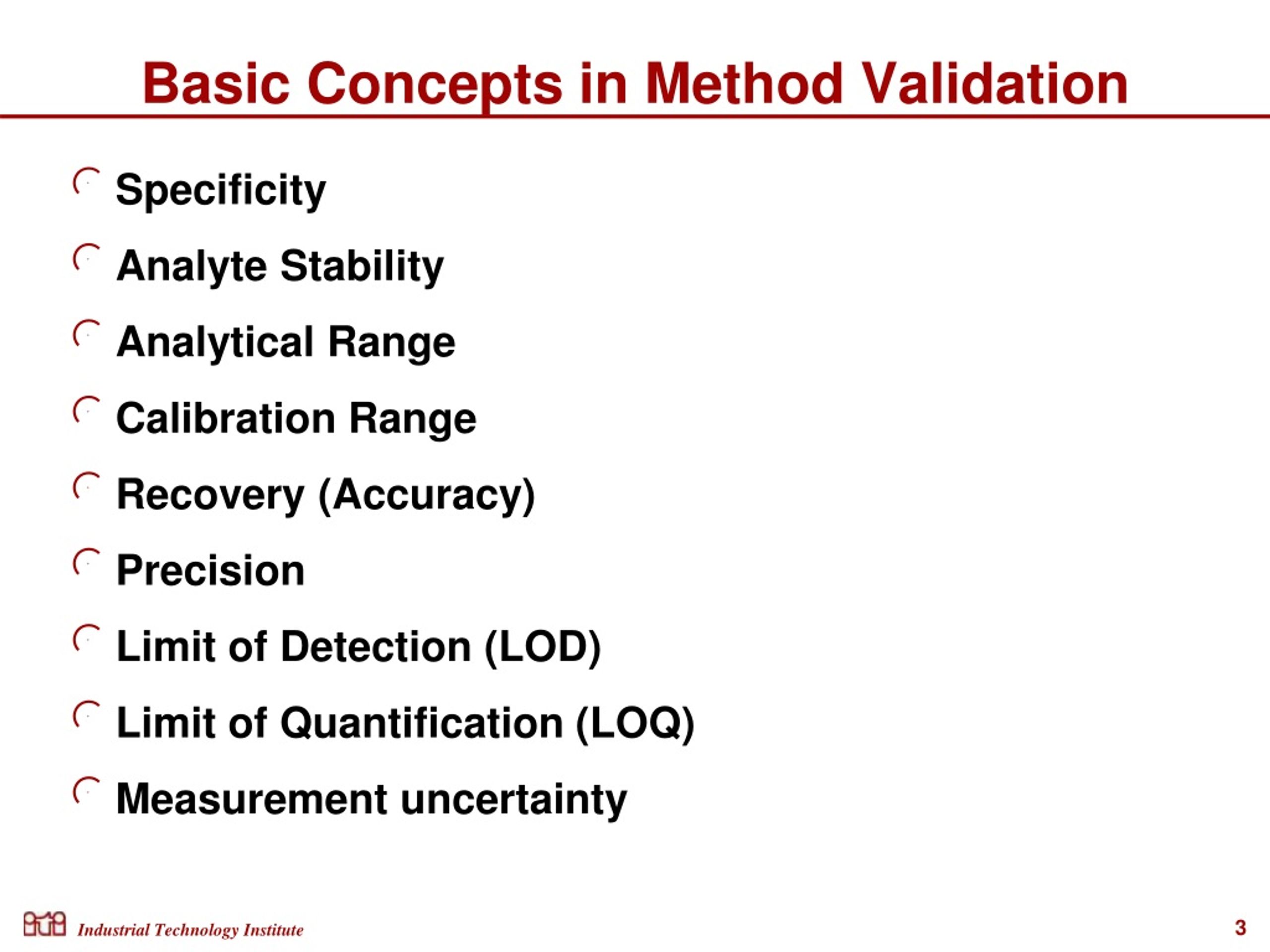
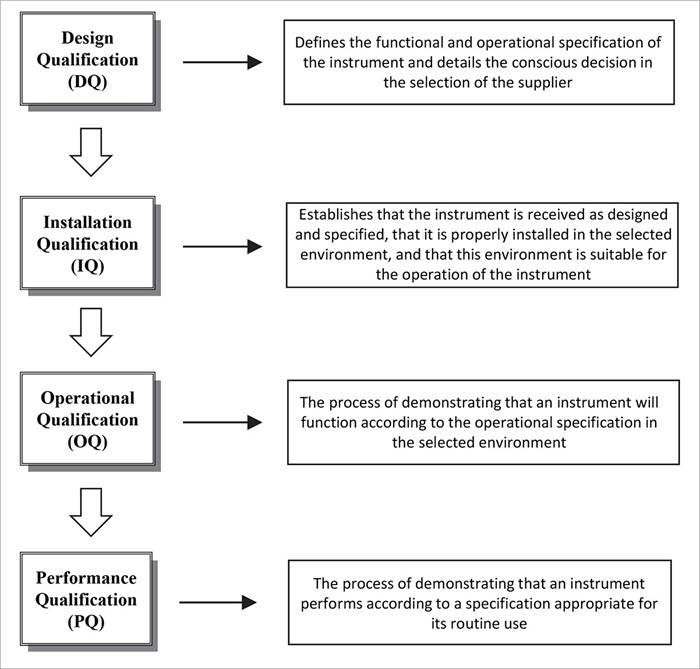
What Is Test Method Validation - Web test validation is the process of gathering evidence to verify that the intended use, interpretations, and results of a test are. Web purpose of test methods validation a validation study is intended to demonstrate that a given analytical. Test method validation is the documented process of ensuring a pharmaceutical test method is suitable for its intended use.
Web purpose of test methods validation a validation study is intended to demonstrate that a given analytical. Test method validation is the documented process of ensuring a pharmaceutical test method is suitable for its intended use. Web test validation is the process of gathering evidence to verify that the intended use, interpretations, and results of a test are.